1.2 Adoptive cellular therapy with T cells expressing the dendritic cell growth factor Flt3L drives epitope spreading and antitumor immunity
https://www.nature.com/articles/s41590-020-0676-7
Year: 2020
Junyun Lai, Sherly Mardiana, Imran G. House, Kevin Sek, Melissa A. Henderson, Lauren Giuffrida, Amanda X. Y. Chen, Kirsten L. Todd, Emma V. Petley, Jack D. Chan, Emma M. Carrington, Andrew M. Lew, Benjamin J. Solomon, Joseph A. Trapani, Katherine Kedzierska, Maximilien Evrard, Stephin J. Vervoort, Jason Waithman, Phillip K. Darcy & Paul A. Beavis
Affiliations with: * Cancer Immunology Program, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia * Sir Peter MacCallum Department of Oncology, University of Melbourne, Parkville, Victoria, Australia
Summary, novelty, and main findings:
Chimeric antigen receptor (CAR)-engineered T cells are effective in recognizing specifically targeted mutated intracellular proteins but face challenges due to MHC restriction, poor solid tumor infiltration, and antigen variability within tumor tissue. Armored T cells engineered to produce flt3l were used to expand intratumoral cDC1s for epitope spreading against a mixed solid tumor.
Fig. 1: Flt3L drives DC proliferation and T cell–dependent rejection of solid tumors.
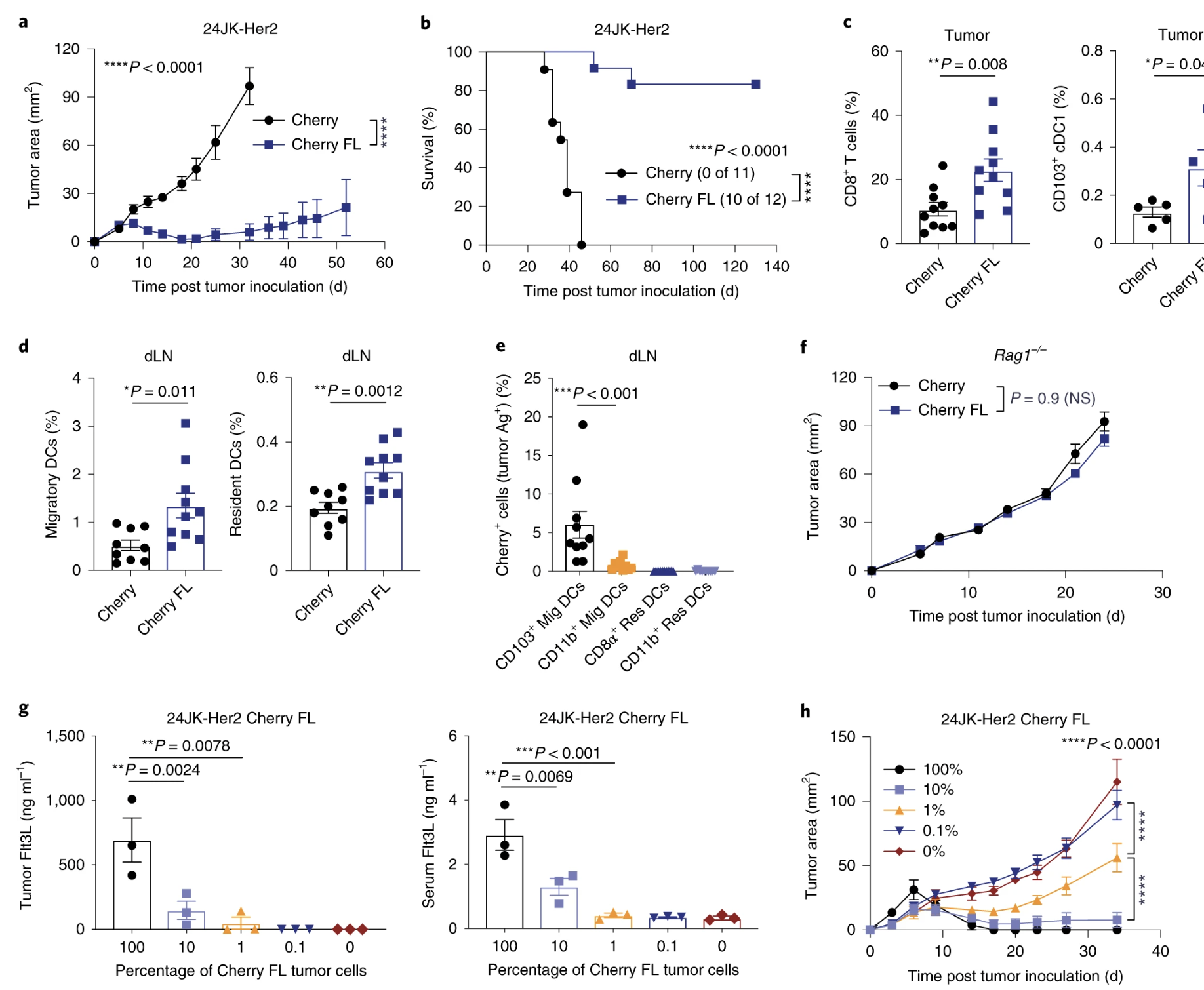
Fig. 2: Flt3L-generated CD103+ DCs induce superior T cell expansion in vitro.
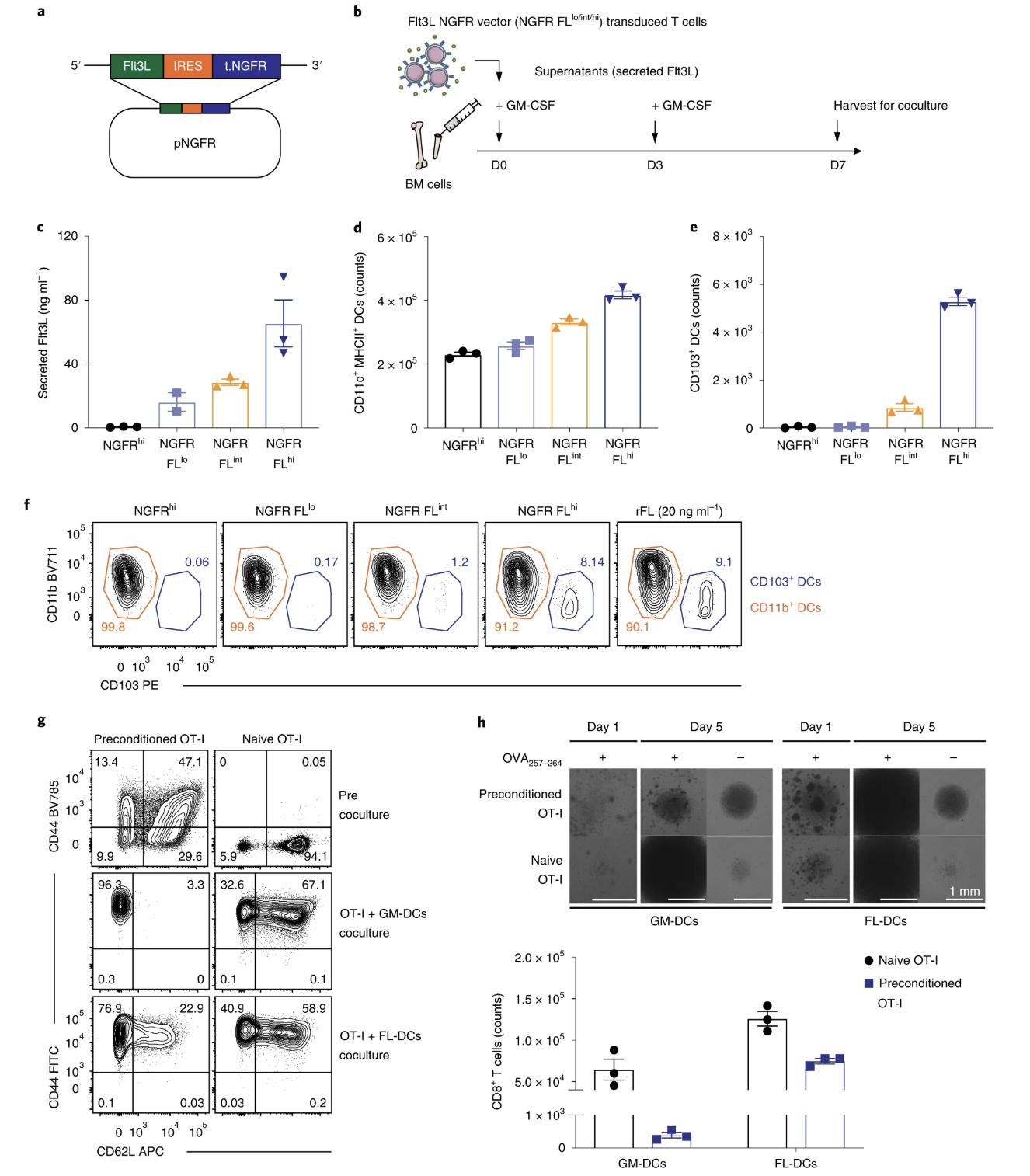
Fig. 3: Adoptive transfer of Flt3L-secreting T cells promotes intratumoral CD103+ cDC1 proliferation and infiltration of endogenous CD8+ T cells.
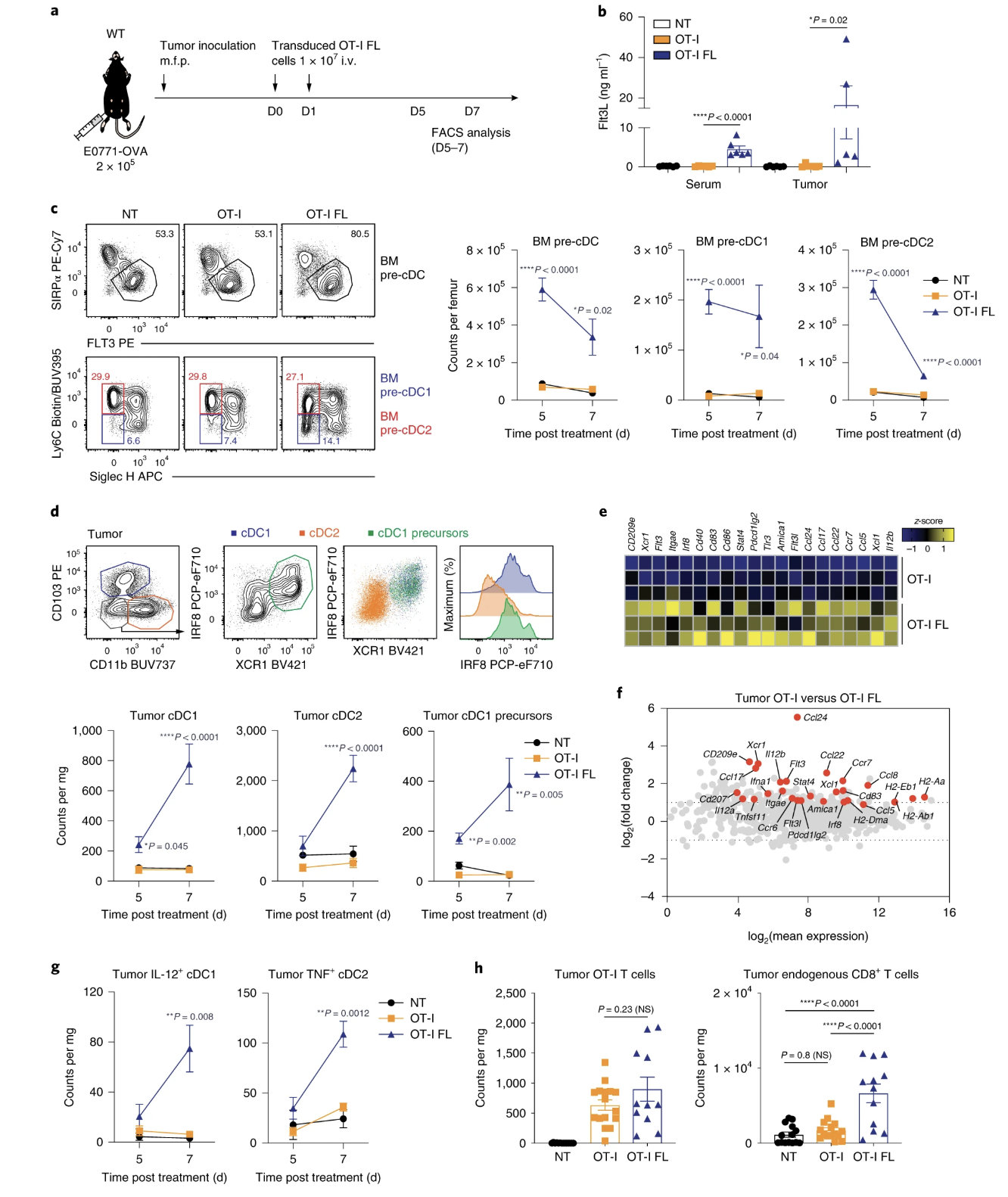
Fig. 4: Flt3L-secreting T cells elicit enhanced inhibition of tumor growth.
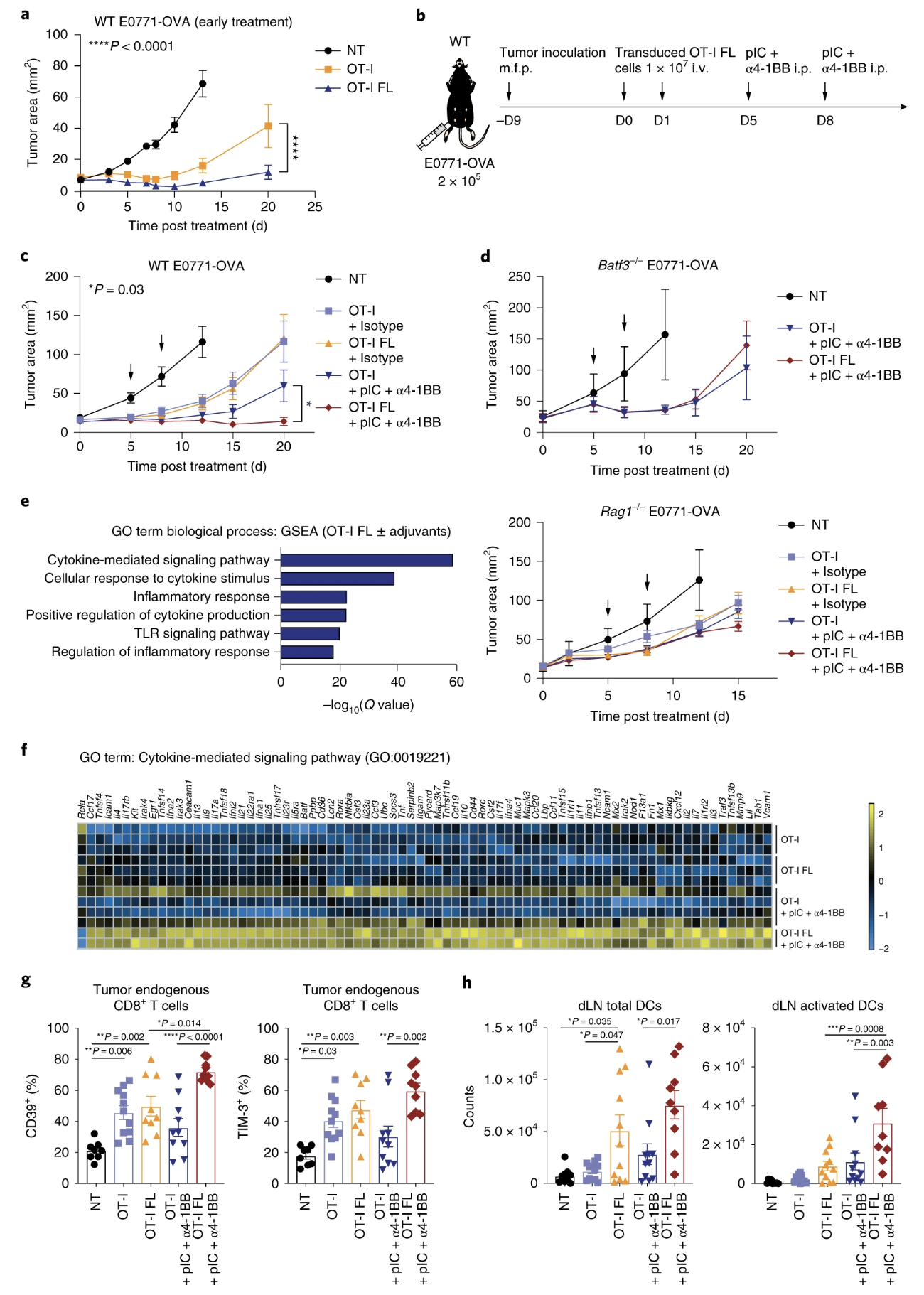
Fig. 5: Flt3L-secreting T cells and immune adjuvants induce oligoclonal expansion of host T cells.
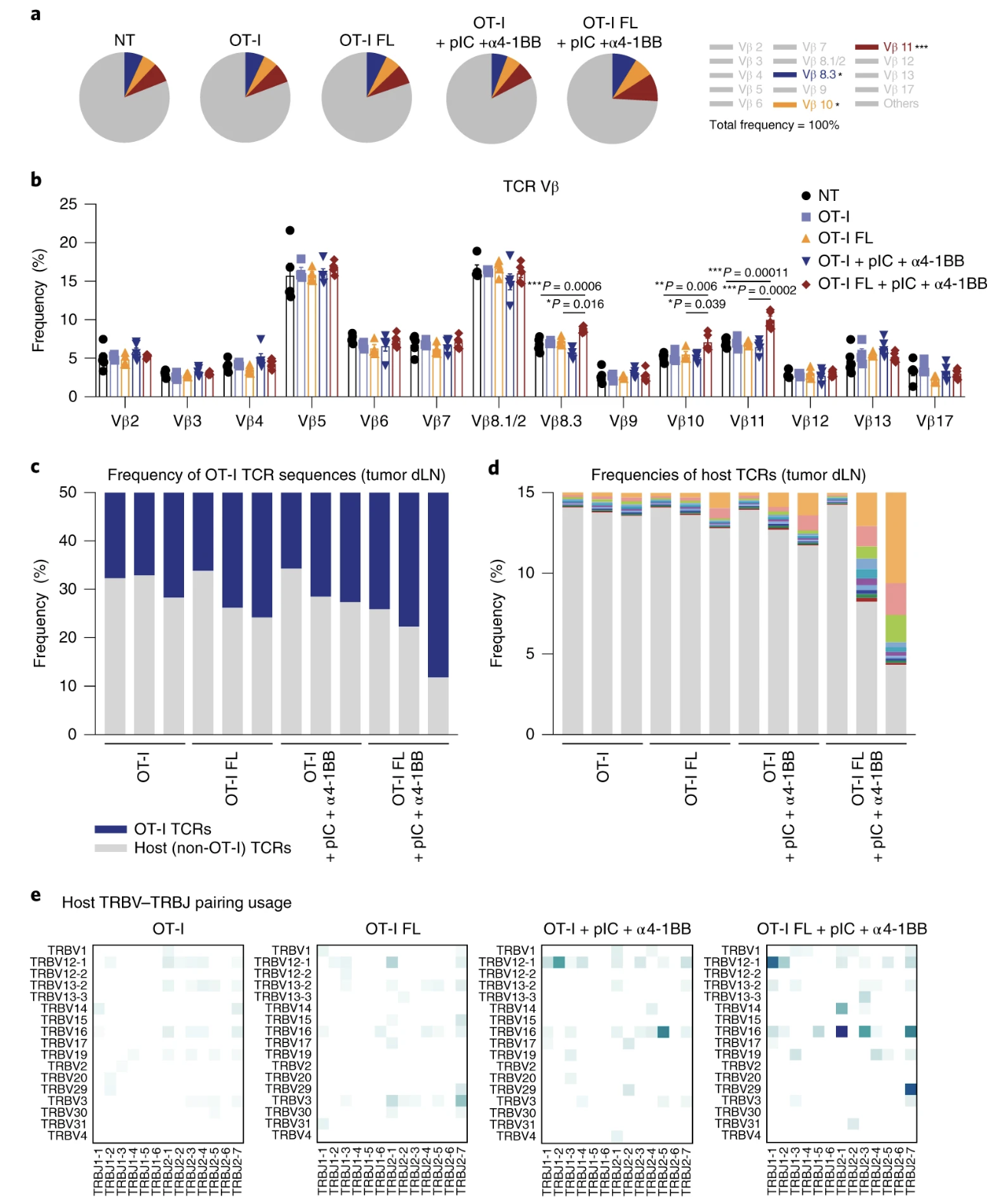
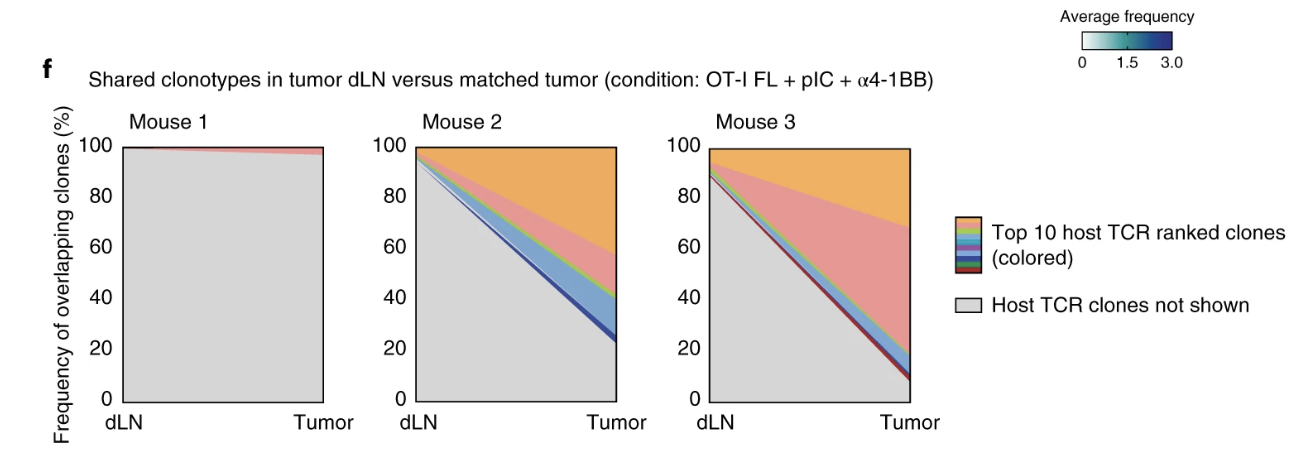
Fig. 6: Flt3L-secreting CAR T cells and immune adjuvants enhance antitumor immunity in a host cDC1- and T cell–dependent manner.
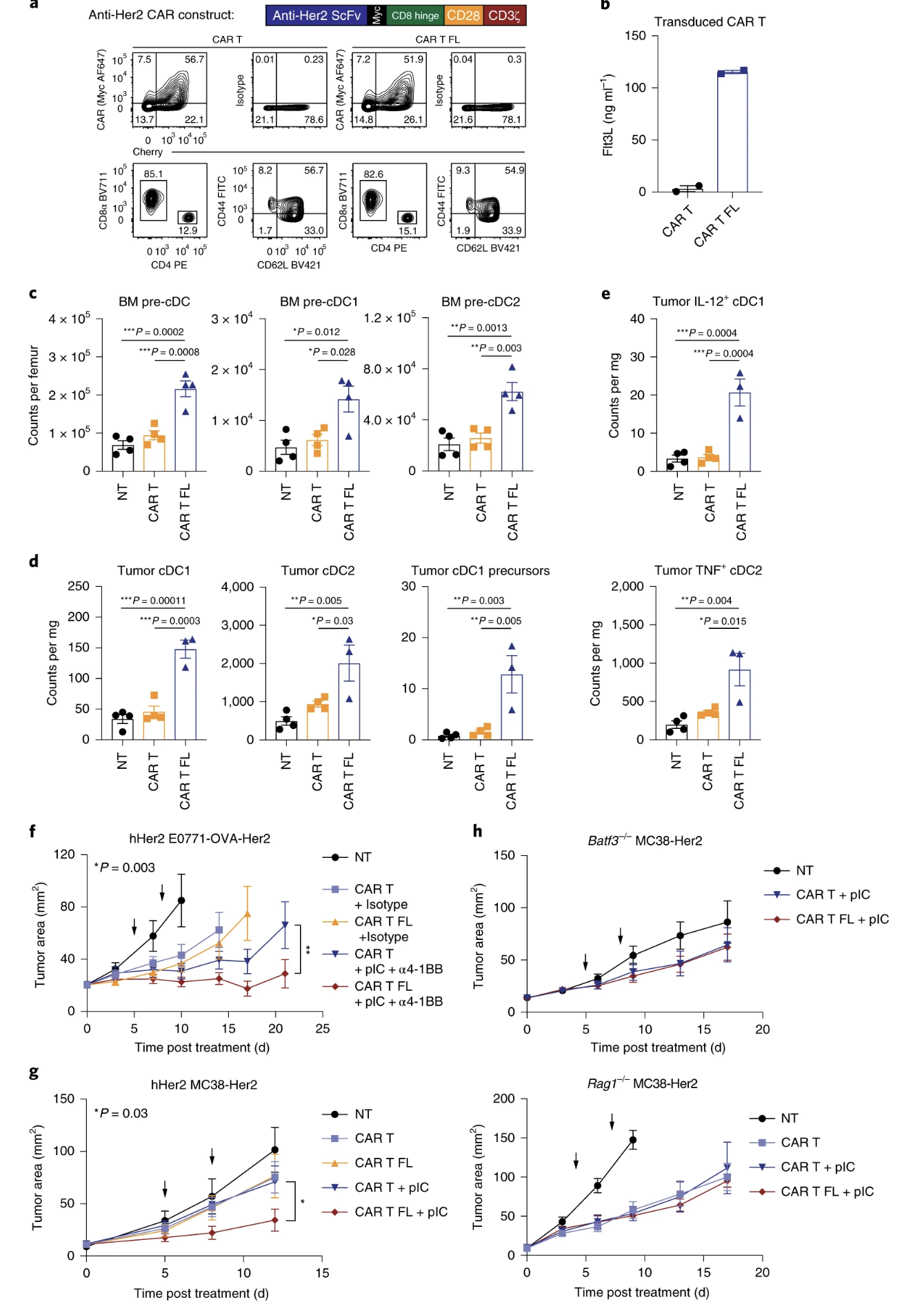
Fig. 7: Combination therapy with Flt3L-secreting CAR T cells induces epitope spreading.
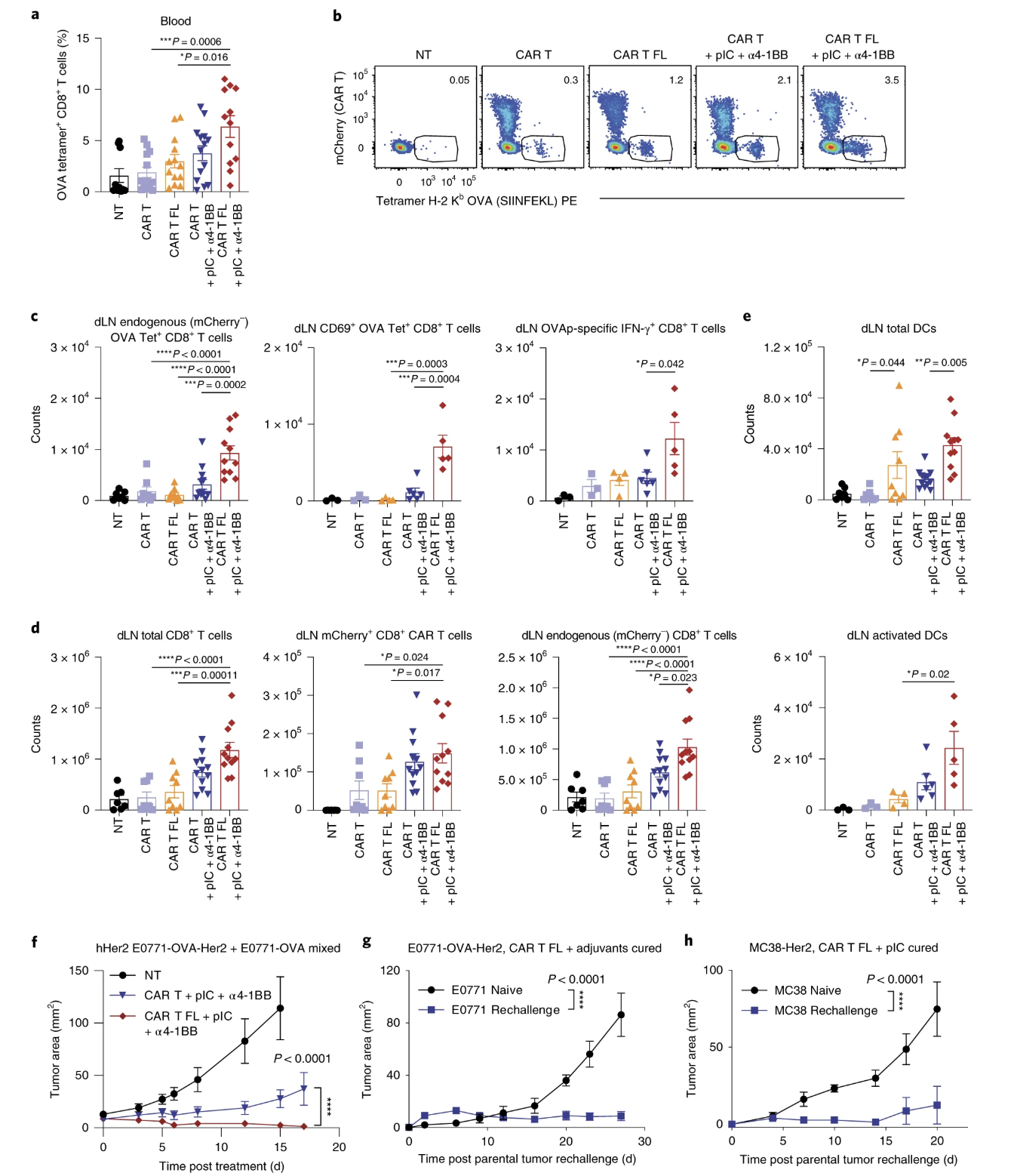
Important Figures: Figures 5, 6, and 7 were important in identifying that flt3l is increasing the diversity/spread of epitopes, that a robust response was possible in a non-immunogenic cancer line, that flt3l driven maturation of DCs was able to clear a mixed tumor, and that flt3l maturation of DCs with adjuvants or poly (I:C) was enough to create memory for tumor rechallenge.
Holes, questions, and future directions:
- Figure 1:
- (1a) What does the data look like if you overexpress flt3l in the cells? This is also pertinent to other figures in their paper (ex. Fig. 3h).
- (1f) Will restoration of rag-/- mice demonstrate a tumor regression phenotype (Lagresile-Peyrou et al. 2006)? However, this is probably difficult.
- Figure 2:
- (2f) Would be stronger to use a proliferation marker, such as ki67, to state that FL-DCs are superior at proliferating rather than assume based on only cell count and visual.
- (1a) What does the data look like if you overexpress flt3l in the cells? This is also pertinent to other figures in their paper (ex. Fig. 3h).
- Figure 4:
- (4d) will restoration of batf3-/- of rag -/- restore antitumor functions?
- (4e) Was a little bit confused on how exactly they did this analysis. Seemed strange until I googled it. Ontology based research is interesting. Would be interesting to see if they can revalidate obtained information with something like sc-rna-seq of the DCs to recapitulate similar information that they obtained through bioinformatics. However, revalidating is probably redundant given that this is an established tool.
- (4e) This set of information seemed weak, the researchers could investigate way more markers to get a better picture of the types of immune cells present. This is a possible future direction to determine immune cell fate.
- Figure 7:
- (7f) I feel like mixing two different types of tumor cells is not enough to say that the experiment mimicked clinical heterogeneity, but probably okay for broadly assessing the ability of the CAR T cells in distinguishing and clearing a mixed tumor.
General question: + What would happen if flt3l knock out cells/mice were challenged with tumor?