1.5 Intratumoral Activity of the CXCR3 Chemokine System Is Required for the Efficacy of Anti-PD-1 Therapy
DOI: https://doi.org/10.1016/j.immuni.2019.04.010
Melvyn T Chow, Aleksandra J Ozga, Rachel L Servis, Dennie T Frederick, Jennifer A Lo, David E Fisher, Gordon J Freeman, Genevieve M Boland, Andrew D Luster
Year: 2019
Affiliations with Harvard Medical School
Figure 1 CXCR3 Is Necessary for Response to Anti-PD-1 Therapy
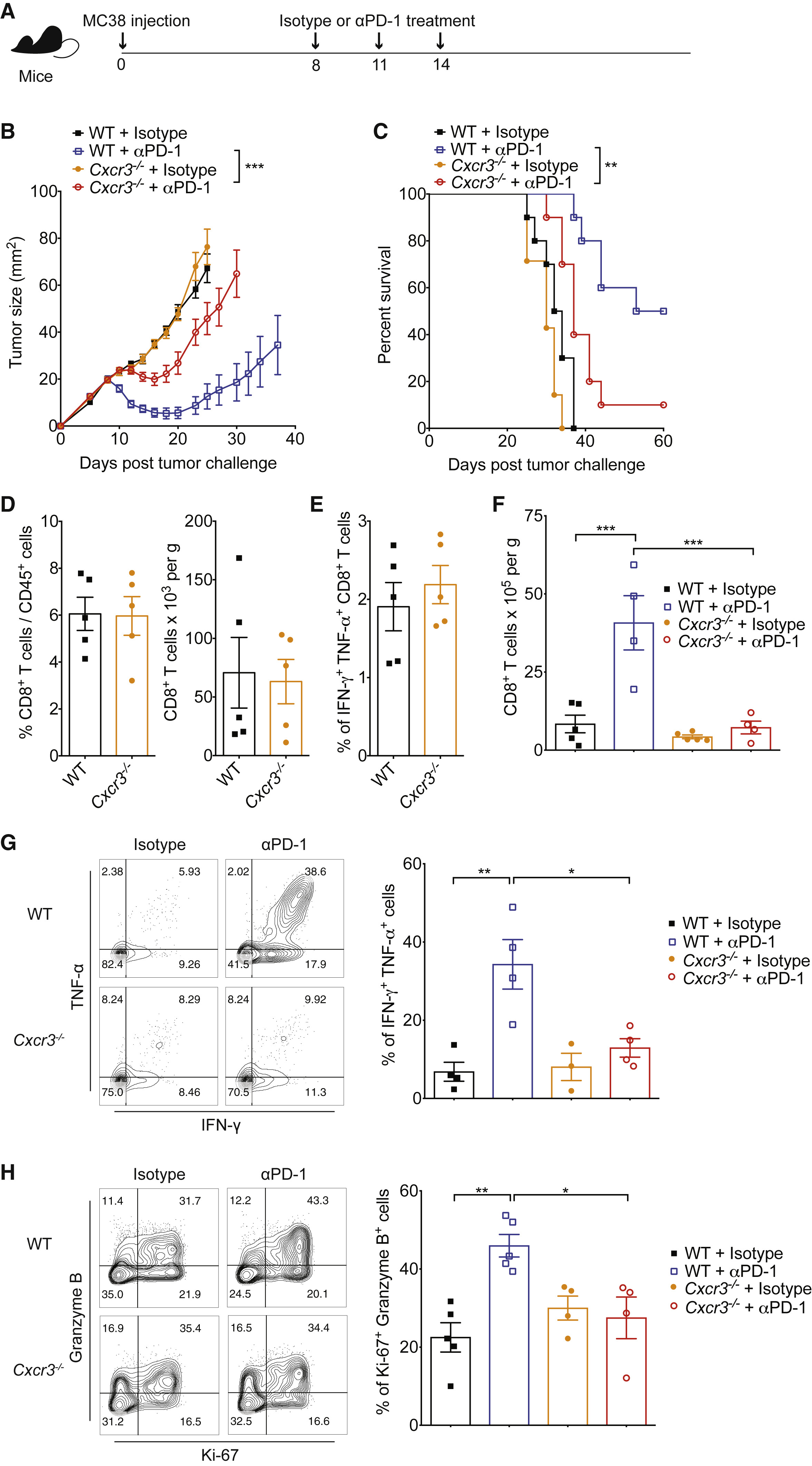
Figure 2 Pre-existing Intratumoral CD8+ T Cell Population Is Sufficient for Anti-PD-1-Induced Tumor Control
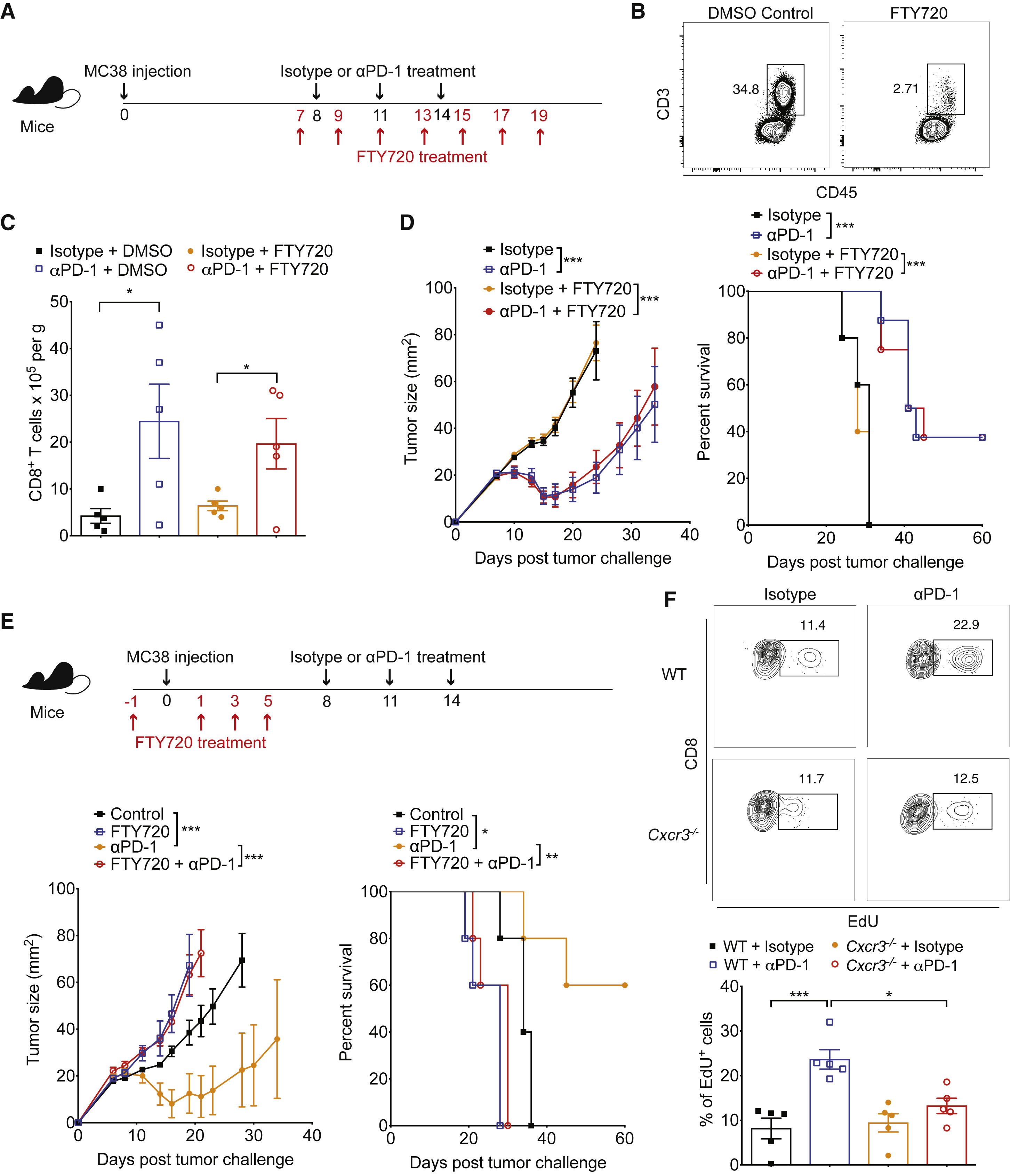
Figure 4 Host-Derived CXCL9 Is Required for Response to Anti-PD-1
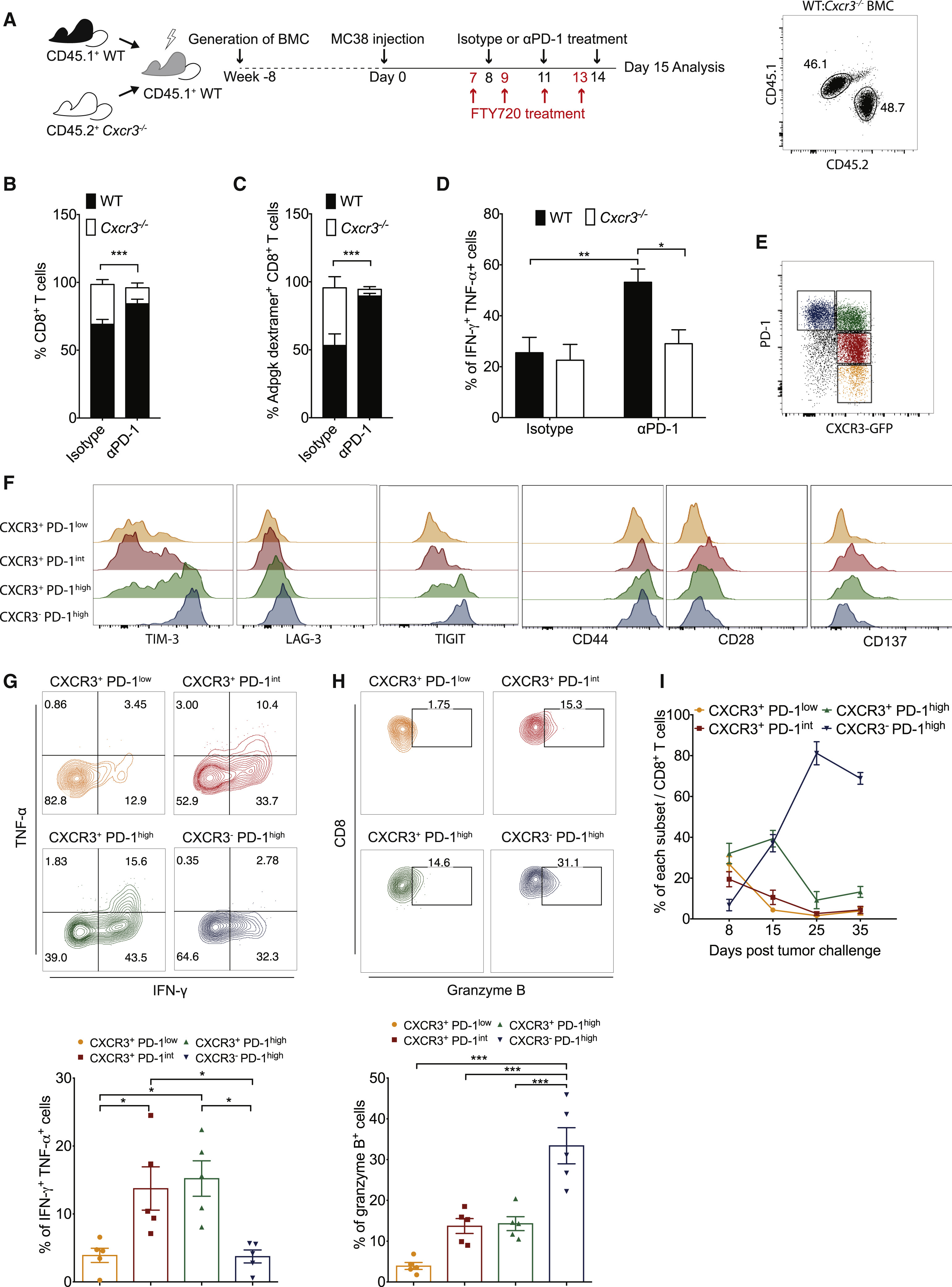
Figure 5 CD103+ DC-Derived CXCL9 Is Important for the Efficacy of PD-1 Blockade
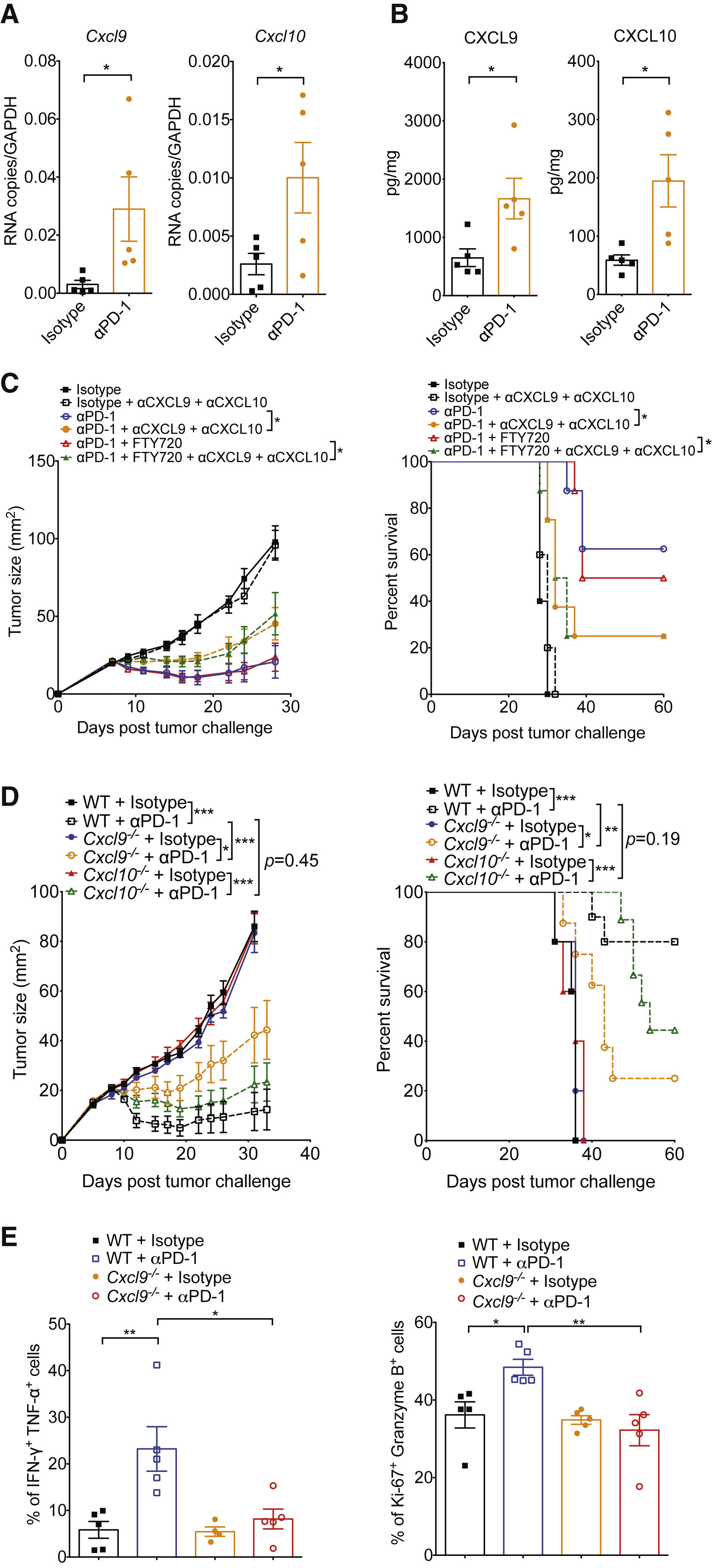
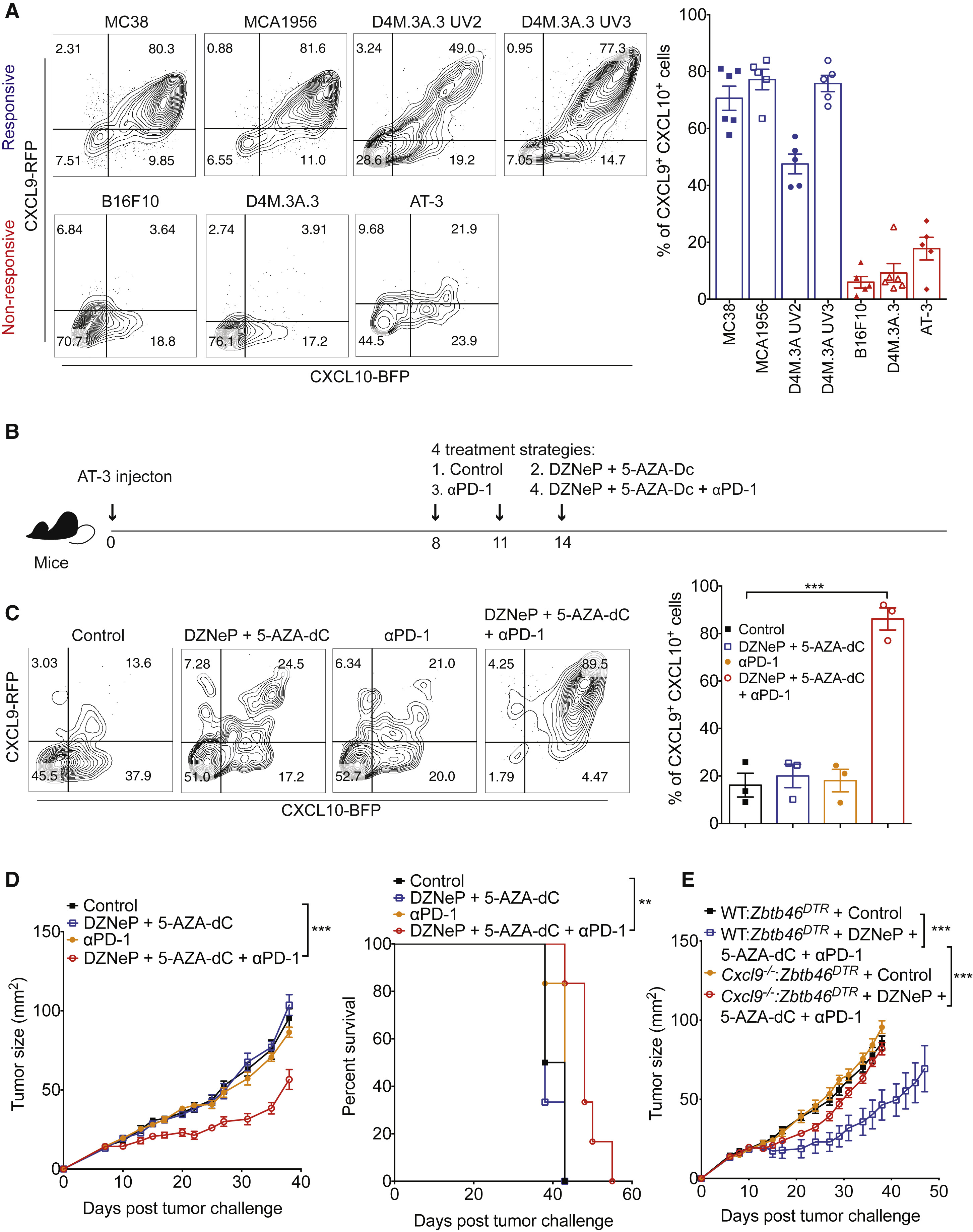
Figure 6 The Combination of Epigenetic Modulators and Anti-PD-1 Increases CXCR3 Ligand Expression and Converts Non-responders to Responders
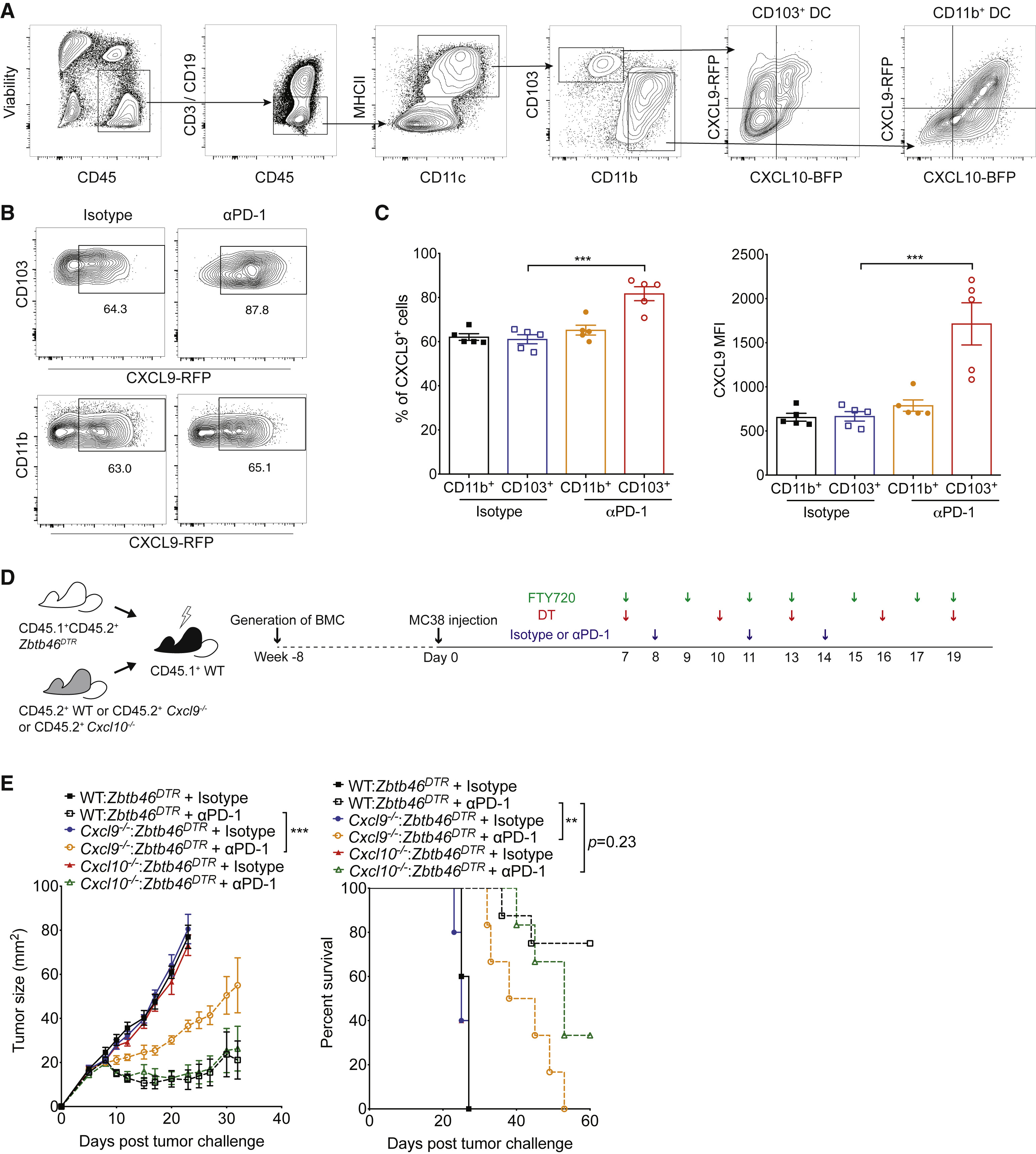
Figure 7 CXCL9 and CXCL10 Expression Correlates with Response to PD-1 Blockade Therapy
Summary, novelty, and main findings:
PD-1 immunotherapy revolutionized immunology by exploiting a conical immune system function to broadly kill a variety of cancers. However, PD-1 immunotherapy only works in a subset of patients, highlighting the need to understand the regulators and activators of the immune system. CXCR3 chemokine was identified as a biomarker for anti-PD-1 treatment. CXCR3 is necessary for an anti-PD-1 response that is not due to the intratumoral CD8 T-cells present (Figure 1 and 2). CXCR3 is necessary for anti-PD-1 CD8 T-cell activation and response (Figure 3). Additionally, the response of CXCR3 deficient mice behaved like exhausted T-cells with expression of exhaustion specific receptors (Figure 3). A chemokine and ligand relationship was identified (CXCR3 on T Cells and CXCL9 on CD103+ DC) in correlation with decreased efficacy of anti-PD-1 treatment (Figure 4, 5, and 6). Non-responding tumors were then epigenetically modulated to become responders to PD-1 immunotherapy (Figure 7).
Most important figures:
The most important figures highlight that CXCL9 CD103+ Dendritic cells play an important part in eliciting a CD8 T-cell tumor response through chemokine CXCR3 (Figure 3) and that the immune system can be epigenetically modulated to perform a CXCL9-CXCLR3 interaction (Figure 6).
Holes of the paper: This paper was thorough, and I had trouble identifying possible holes or assumptions.
Future directions for the paper:
This paper highlights the importance of finding costimulatory regulators of PD-1. Future directions for this paper may not be directly related to CXCR3, CXCL9, or CXCL10, but to identify other stimulatory molecules that work in conjunction with PD-1 for combination immunotherapy.